The
green sulfur bacteria (Chlorobiaceae) are
photosynthetic bacteria. The Chlorobiaceae form a coherent group that is phylogenetically isolated from all other microbes, so they are the sole occupants of their
phylum (
Phylum Chlorobi). There are 5 known genera and 14 species in the family Chlorobiaceae with the type strain being Chlorobium limicola.
Phylum Bacteroidetes comprise the most closely related phylum.
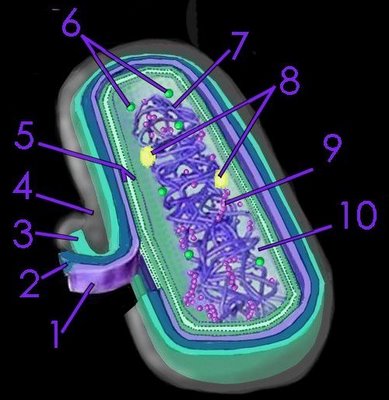
Chlorobiaceae are anaerobic obligate photoautolithotrophs that use sulfide, elemental sulfur or hydrogen as their source of electrons, employing
bacteriochlorophylls c, d, and e in
vesicles called
chlorosomes that are attached to the membrane. As anaerobes, their environment must be oxygen-free, and, as phototrophs, they require light as an energy source. For example, in conducting
nonoxygenic photosynthesis employing sulfide ions (H
2S) as an electron donor:
CO2 + 2H2S = CH2O + H2O + 2S
The sulfide donates an electron, becoming oxidized and producing globules of elemental sulfur outside the cell, which may then be further oxidized. (By contrast,
oxygenic photosynthesis in plants employs water as electron donor and produces oxygen.) Green sulfur bacteria form associations with other microbes that are beneficial both species. When green bacteria convert sulfide to elemental sulfur they store it externally. Some
purple bacteria can associate with the green sulfur bacteria and oxidize the sulfur for their own photosynthetic processes. In this relationship, the green sulfur bacteria has a high affinity for sulfide and detoxifies it for the purple bacteria, while the purple bacteria remove the sulfur end product, driving the metabolism of the green sulfur bacteria. There are also examples of green bacteria associating with chemoautolithotrophic bacteria and forming what are called phototrophic consortia in which the green sulfur bacteria form a regular array around a central colorless bacterial cell.
Chlorobiaceae:
(chlorophylls mainly bchl c, d or e)
electron donor for photoautotrophy = S– or S
o (S
o globules formed outside cell from S–)
photoheterotrophy? potentially all spp. chemotrophy? none
Due to their requirement for an oxygen-free environment and for sulfide and light, the green sulfur bacteria are restricted to narrow zones in aquatic environments where gradients of light and sulfide overlap under anaerobic conditions. In lakes this narrow zone occurs at depths of 2 to 20 m depending upon the characteristics of the body of water. The Chlorobiaceae also exist in microbial mats, which are communities with different classes of photosynthetic bacteria.
Cyanobacteria are often on the top layer,
purple sulfur bacteria next and the green sulfur bacteria are on the bottom. This relationship relegates the Chlorobiaceae cells to low light intensities, but minimizes oxygen exposure. The green sulfur bacteria possess highly efficient light-harvesting structures called
chlorosomes, which confer their ability to operate at extremely low light intensities .
A species of green sulfur bacteria has been found living near a
black smoker off the coast of Mexico at a depth of 2,500 meters beneath the surface of the Pacific Ocean. At this depth, where no sunlight can penetrate, the bacteria, designated GSB1, utilizes the dim glow of the thermal vent.
Green sulfur bacteria are typically nonmotile since only one species is
flagellated. Morphologically, green sulfur bacteria are gram-negative rods, vibrio or have a coccus morhphology. Green sulfur bacteria of the
genus Prothecocholoris are spherical with a prosthecae emerging from the sphere. Prosthecae have been postulated to be extra
membrane areas that can be filled with additional photosynthetic units. Prosthecae are characteristic appendages that give the cells a star-like shape, and are of a different nature than other external appendages, such as spinae.